
Menveo: Meningococcal (Groups A, C, Y and W-135) Oligosaccharide Diphtheria CRM197 Conjugate Vaccine: patient information, prescribing information, ingredients, manufacturer, adverse reactions and side effects
Tuesday, April 11, 2017 by Gregory Van Dyke
http://www.naturalnewsreference.com/2017-04-11-menveo-meningococcal-groups-a-c-y-and-w-135-oligosaccharide-diphtheria-crm197-conjugate-vaccine-patient-information-prescribing-information-ingredients-manufacturer-adverse-reactions-and-sid.html
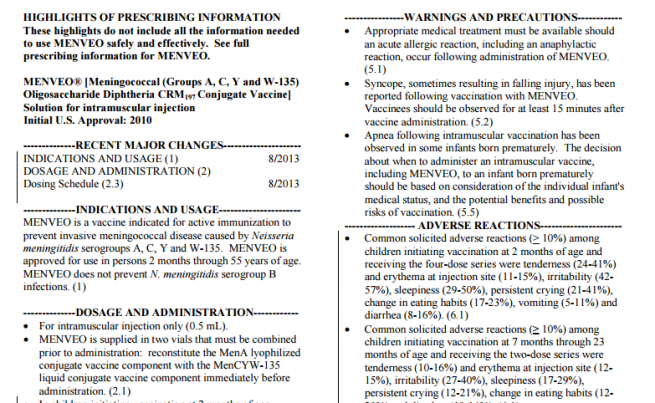
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use MENVEO safely and effectively. See full prescribing information for MENVEO.
See full insert sheet at this link at the Natural News Reference website.
MENVEO® [Meningococcal (Groups A, C, Y and W-135) Oligosaccharide Diphtheria CRM197 Conjugate Vaccine]
Solution for intramuscular injection
Initial U.S. Approval: 2010
INGREDIENTS AND EXCIPIENTS
MENVEO [Meningococcal (Groups A, C, Y and W-135) Oligosaccharide Diphtheria CRM197 Conjugate Vaccine] is a sterile liquid vaccine administered by intramuscular injection that contains N. meningitidis serogroup A, C, Y and W-135 oligosaccharides conjugated individually to Corynebacterium diphtheriae CRM197 protein. The polysaccharides are produced by bacterial fermentation of N. meningitidis (serogroups A, C, Y or W-135). N. meningitidis strains A, C, Y and W-135 are each cultured and grown on Franz Complete medium and treated with formaldehyde. MenA, MenW- 135 and MenY polysaccharides are purified by several extraction and precipitation steps. MenC polysaccharide is purified by a combination of chromatography and precipitation steps.
The protein carrier (CRM197) is produced by bacterial fermentation and is purified by a series of chromatography and ultrafiltration steps. C. diphtheriae is cultured and grown on CY medium containing yeast extracts and amino acids. The oligosaccharides are prepared for conjugation from purified polysaccharides by hydrolysis, sizing, and reductive amination. After activation, each oligosaccharide is covalently linked to the CRM197 protein. The resulting glycoconjugates are purified to yield the four drug substances, which compose the final vaccine. The vaccine contains no preservative or adjuvant. Each dose of vaccine contains 10 µg MenA oligosaccharide, 5 µg of each of MenC, MenY and MenW-135 oligosaccharides and 32.7 to 64.1 µg CRM197 protein. Residual formaldehyde per dose is estimated to be not more than 0.30 µg.
The vials in which the vaccine components are contained are composed of Type I glass, USP. The container closures (synthetic rubber stoppers) do not contain latex.
INDICATIONS AND USAGE
MENVEO is a vaccine indicated for active immunization to prevent invasive meningococcal disease caused by Neisseria meningitidis serogroups A, C, Y and W-135. MENVEO is approved for use in persons 2 months through 55 years of age. MENVEO does not prevent N. meningitidis serogroup B infections. (1)
DOSAGE AND ADMINISTRATION
For intramuscular injection only (0.5 mL).
MENVEO is supplied in two vials that must be combined prior to administration: reconstitute the MenA lyophilized conjugate vaccine component with the MenCYW-135 liquid conjugate vaccine component immediately before administration. (2.1)
In children initiating vaccination at 2 months of age, MENVEO is to be administered as a four-dose series at 2, 4, 6, and 12 months of age. (2.3)
In children initiating vaccination at 7 months through 23 months of age, MENVEO is to be administered as a twodose series with the second dose administered in the second year of life and at least three months after the first dose. (2.3)
In individuals 2 years through 55 years of age Menveo is to be administered as a single dose. (2.3)
DOSAGE FORMS AND STRENGTHS
Solution for intramuscular injection supplied as a lyophilized Men A conjugate vaccine component to be reconstituted with the accompanying Men CYW-135 liquid conjugate vaccine component. A single dose, after reconstitution is 0.5 mL (3)
CONTRAINDICATIONS
Severe allergic reaction (e.g., anaphylaxis) after a previous dose of MENVEO, any component of this vaccine, or any other CRM197, diphtheria toxoid or meningococcal-containing vaccine is a contraindication to administration of MENVEO. (4)
WARNINGS AND PRECAUTIONS
Appropriate medical treatment must be available should an acute allergic reaction, including an anaphylactic reaction, occur following administration of MENVEO. (5.1)
Syncope, sometimes resulting in falling injury, has been reported following vaccination with MENVEO. Vaccinees should be observed for at least 15 minutes after vaccine administration. (5.2)
Apnea following intramuscular vaccination has been observed in some infants born prematurely. The decision about when to administer an intramuscular vaccine, including MENVEO, to an infant born prematurely should be based on consideration of the individual infant’s medical status, and the potential benefits and possible risks of vaccination. (5.5)
ADVERSE REACTIONS
Common solicited adverse reactions (> 10%) among children initiating vaccination at 2 months of age and receiving the four-dose series were tenderness (24-41%) and erythema at injection site (11-15%), irritability (42- 57%), sleepiness (29-50%), persistent crying (21-41%), change in eating habits (17-23%), vomiting (5-11%) and diarrhea (8-16%). (6.1)
Common solicited adverse reactions (≥ 10%) among children initiating vaccination at 7 months through 23 months of age and receiving the two-dose series were tenderness (10-16%) and erythema at injection site (12- 15%), irritability (27-40%), sleepiness (17-29%), persistent crying (12-21%), change in eating habits (12- 20%) and diarrhea (10-16%). (6.1)
Common solicited adverse reactions (> 10%) among children 2 years through 10 years of age who received MENVEO were injection site pain (31%), erythema (23%), irritability (18%), induration (16%), sleepiness (14%), malaise (12%), and headache (11%). (6.1)
Common solicited adverse reactions (> 10%) among adolescents and adults who received MENVEO were pain at the injection site (41%), headache (30%), myalgia (18%), malaise (16%) and nausea (10%). (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Novartis Vaccines at 1-877-683-4732 or VAERS at 1-800- 822-7967 or http://vaers.hhs.gov.
DRUG INTERACTIONS
Do not mix MENVEO or any of its components with any other vaccine or diluent in the same syringe or vial. (7.1)
USE IN SPECIFIC POPULATIONS
Pregnancy
Pregnancy Category B
Reproduction studies have been performed in female rabbits at a dose of approximately 20 times the human dose (on a mg/kg basis) and have revealed no evidence of impaired fertility or harm to the fetus due to MENVEO. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, MENVEO should be given to a pregnant woman only if clearly needed.
Nonclinical Studies
The effect of MENVEO on embryo-fetal and post-natal development was evaluated in pregnant rabbits. Animals were administered MENVEO 3 times prior to gestation, during the period of organogenesis (gestation day 7) and later in pregnancy (gestation day 20), 0.5 mL/rabbit/occasion (approximately 20-fold excess relative to the projected human dose on a body weight basis) by intramuscular injections. There were no adverse effects attributable to the vaccine on mating, female fertility, pregnancy, or embryo-fetal development. There were no vaccine related fetal malformations or other evidence of teratogenesis noted in this study.
Clinical Studies
To date, no clinical trials have been specifically designed to evaluate the use of MENVEO in pregnant or lactating women.
Among the 5065 women in the adolescent and adult populations enrolled in the studies, 43 women were found to be pregnant during the 6-month follow-up period after vaccination. Of these, 37 pregnancies occurred among 3952 MENVEO recipients (7 spontaneous abortions, no congenital anomalies). Six pregnancies occurred among 1113 Menactra recipients (no spontaneous abortions, one congenital anomaly (hydrocephalus)).
Among the seven subjects with adverse pregnancy outcomes who had received MENVEO, the estimated dates of conception were 5 days prior to vaccination (1 subject), 6 to 17 weeks post vaccination (5 subjects), and 6 months post vaccination (1 subject). In the subject who had received Menactra the estimated date of conception was approximately 15 weeks post immunization.
Safety and effectiveness of MENVEO have not been established in pregnant women.
Pregnancy Registry for MENVEO
Novartis Vaccines and Diagnostics Inc. maintains a pregnancy registry to monitor the fetal outcomes of pregnant women exposed to MENVEO. To enroll in the Novartis Vaccines and Diagnostics Inc. pregnancy registry, please call +1 877-413-4759.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when MENVEO is administered to a nursing woman. No studies have been conducted to assess the impact of MENVEO on milk production, its presence in breast milk or its effects on the breastfed child.
Pediatric Populations
Safety and effectiveness of MENVEO in children under 2 months of age have not been established.
Geriatric Populations
Safety and effectiveness of MENVEO in adults 65 years of age and older have not been established.
Revised: 08/2013
https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM201349.pdf
Tagged Under: Tags: dosage, ingredients, insert sheet, menveo, side effects, usage, warnings





