
BCG Live Vaccine: Bacillus of Calmette and Guerin (BCG) strain of Mycobacterium bovis: patient information, prescribing information, ingredients, manufacturer, adverse reactions and side effects
Friday, April 07, 2017 by Gregory Van Dyke
http://www.naturalnewsreference.com/2017-04-07-bcg-live-vaccine-bacillus-of-calmette-and-guerin-bcg-strain-of-mycobacterium-bovis-patient-information-prescribing-information-ingredients-manufacturer-adverse-reactions-and-side-effects.html
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use BGC VACCINE safely and effectively. See full prescribing information for BGC VACCINE.
See full insert sheet at this link at the Natural News Reference website.
BGC VACCINE
Suspension for Intradermal Injection
Initial US Approval:
INGREDIENTS AND EXCIPIENTS
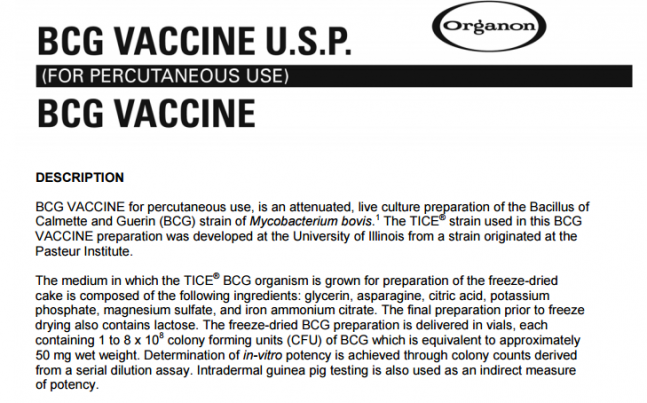
BCG VACCINE for percutaneous use, is an attenuated, live culture preparation of the Bacillus of Calmette and Guerin (BCG) strain of Mycobacterium bovis. 1 The TICE® strain used in this BCG VACCINE preparation was developed at the University of Illinois from a strain originated at the Pasteur Institute.
The medium in which the TICE® BCG organism is grown for preparation of the freeze-dried cake is composed of the following ingredients: glycerin, asparagine, citric acid, potassium phosphate, magnesium sulfate, and iron ammonium citrate. The final preparation prior to freeze drying also contains lactose. The freeze-dried BCG preparation is delivered in vials, each containing 1 to 8 x 108 colony forming units (CFU) of BCG which is equivalent to approximately 50 mg wet weight. Determination of in-vitro potency is achieved through colony counts derived from a serial dilution assay. Intradermal guinea pig testing is also used as an indirect measure of potency.
Reconstitution requires addition of Sterile Water for Injection, U.S.P. at 4–25°C (39–77°F). For an adult dosage, 1 mL of Sterile Water for Injection, U.S.P., should be added to one vial of vaccine. For a pediatric dosage, 2 mL of Sterile Water for Injection, U.S.P., should be added to one vial of vaccine (see DOSAGE AND ADMINISTRATION).
INDICATIONS AND USAGE
BCG VACCINE (TICE® strain) is indicated for the prevention of tuberculosis in persons not previously infected with M. tuberculosis who are at high risk for exposure. As with any vaccine, immunization with BCG VACCINE may not protect 100% of susceptible individuals.
DOSAGE AND ADMINISTRATION
Using aseptic methods, 1 mL of Sterile Water for Injection, U.S.P. at 4–25°C (39–77°F), is added to one vial of vaccine.
Pediatric Dose
Do not administer INTRAVENOUSLY, SUBCUTANEOUSLY, INTRAMUSCULARLY OR INTRADERMALLY. Administer the vaccine in the deltoid region.
In infants less than 1 month old, the dosage of BCG VACCINE should be reduced by one-half, by using 2 mL of Sterile Water for Injection, U.S.P. at 4–25°C (39–77°F) when reconstituting. If a vaccinated infant remains tuberculin negative to 5 TU on skin testing, and if indications for vaccination persist, the infant should receive a full dose after 1 year of age.
DOSAGE FORMS AND STRENGTHS
BCG VACCINE is supplied in a box of one vial of BCG. Each vial contains 1 to 8 x 108 CFU, which is equivalent to approximately 50 mg (wet weight), as lyophilized (freeze-dried) powder, NDC 0052-0603-02.
CONTRAINDICATIONS
BCG VACCINE for prevention of tuberculosis should not be given to persons (a) whose immunologic responses are impaired because of HIV infections, congenital immunodeficiency such as chronic granulomatous disease or interferon gamma receptor deficiency, leukemia, lymphoma, or generalized malignancy or (b) whose immunologic responses have been suppressed by steroids, alkylating agents, antimetabolites, or radiation.3 BCG VACCINE should not be administered to HIV-infected or immunocompromised infants, children, or adults.
Prior to administration, the possibility of allergic reactions should be assessed. Allergy to any component of BCG VACCINE or an anaphylactic or allergic reaction to a previous dose of BCG VACCINE are contraindications for vaccination.
BCG VACCINE is not a vaccine for the treatment of active tuberculosis.
BCG VACCINE should not be used in infants, children, or adults with severe immune deficiency syndromes. Children with a family history of immune deficiency disease should not be vaccinated; if they are, an infectious disease specialist should be consulted and antituberculous therapy administered if clinically indicated.18
WARNINGS AND PRECAUTIONS
Administration should be by the percutaneous route with the multiple puncture device as described below. DO NOT INJECT INTRAVENOUSLY, SUBCUTANEOUSLY, INTRAMUSCULARLY OR INTRADERMALLY.
ADVERSE REACTIONS
Although BCG vaccination often causes local reactions, serious or long-term complications are rare.3
All suspected adverse reactions to BCG vaccination should be reported to Organon USA Inc. at (800) 842-3220 and to the Vaccine Adverse Effect Reporting System (VAERS); telephone (800) 822-7967. These reactions occasionally could occur more than 1 year after vaccination.
DRUG INTERACTIONS
Antimicrobial or immunosuppressive agents may interfere with the development of the immune response and should be used only under medical supervision.
USE IN SPECIFIC POPULATIONS
Pregnancy
Pregnancy Category C: Animal reproduction studies have not been conducted with BCG VACCINE. It is also not known whether BCG VACCINE can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. Although no harmful effects to the fetus have been associated with BCG VACCINE, its use is not recommended during pregnancy.3
Nursing Mothers
It is not known whether BCG is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions from BCG in nursing infants, a decision should be made whether to discontinue nursing or not to vaccinate, taking into account the importance of tuberculosis vaccination to the mother.
Pediatric Use
See Treatment and Schedule under DOSAGE AND ADMINISTRATION section. Precautions should be taken with respect to infants vaccinated with BCG and exposed to persons with active tuberculosis.21
Geriatric Use
Clinical studies of BCG VACCINE did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in response between elderly and younger patients. An intact immune system is a prerequisite for BCG vaccination. If the immune status of an elderly patient, or any patient, is in question, the BCG vaccination should be held until the immune status of the patient has been evaluated.
Revised:
https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM202934.pdf
http://naturalnewsreference.com/vaccine-insert-sheets/BCG-Live-Vaccine.pdf
Tagged Under: Tags: BCG Live Vaccine, dosage, ingredients, insert sheet, side effects, usage, warnings





