
Daptacel: Diphtheria and Tetanus Toxoids and Acellular Pertussis Vaccine Adsorbed: patient information, prescribing information, ingredients, manufacturer, adverse reactions and side effects
Friday, April 07, 2017 by Gregory Van Dyke
http://www.naturalnewsreference.com/2017-04-07-daptacel-diphtheria-and-tetanus-toxoids-and-acellular-pertussis-vaccine-adsorbed-patient-information-prescribing-information-ingredients-manufacturer-adverse-reactions-and-side-effects.html
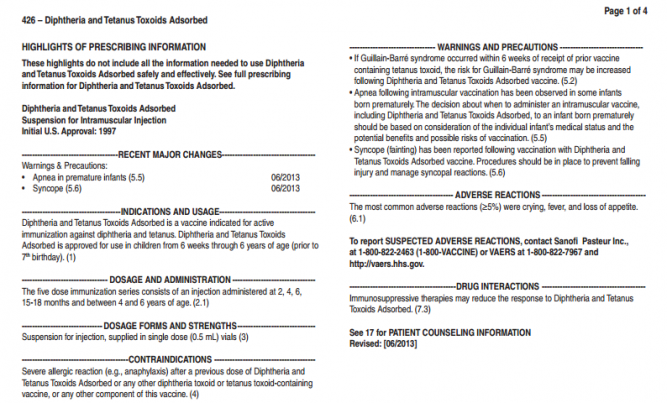
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use DAPTACEL safely and effectively. See full prescribing information for DAPTACEL.
See full insert sheet at this link at the Natural News Reference website.
DAPTACEL (Diphtheria and Tetanus Toxoids and Acellular Pertussis Vaccine Adsorbed)
Suspension for Intramuscular Injection
Initial U.S. Approval: 2002
INGREDIENTS AND EXCIPIENTS
DAPTACEL is a sterile isotonic suspension of pertussis antigens and diphtheria and tetanus toxoids adsorbed on aluminum phosphate, for intramuscular injection.
Each 0.5 mL dose contains 15 Lf diphtheria toxoid, 5 Lf tetanus toxoid and acellular pertussis antigens [10 mcg detoxified pertussis toxin (PT), 5 mcg filamentous hemagglutinin (FHA), 3 mcg pertactin (PRN), and 5 mcg fimbriae types 2 and 3 (FIM)].
Other ingredients per 0.5 mL dose include 1.5 mg aluminum phosphate (0.33 mg of aluminum) as the adjuvant, ≤5 mcg residual formaldehyde, <50 ng residual glutaraldehyde and 3.3 mg (0.6% v/v) 2-phenoxyethanol (not as a preservative).
The acellular pertussis vaccine components are produced from Bordetella pertussis cultures grown in Stainer-Scholte medium (2) modified by the addition of casamino acids and dimethyl-betacyclodextrin. PT, FHA and PRN are isolated separately from the supernatant culture medium. The FIM components are extracted and co-purified from the bacterial cells. The pertussis antigens are purified by sequential filtration, salt-precipitation, ultrafiltration and chromatography. PT is detoxified with glutaraldehyde. FHA is treated with formaldehyde, and the residual aldehydes are removed by ultrafiltration. The individual antigens are adsorbed separately onto aluminum phosphate.
Corynebacterium diphtheriae is grown in modified Mueller’s growth medium. (3) After purification by ammonium sulfate fractionation, diphtheria toxin is detoxified with formaldehyde and diafiltered. Clostridium tetani is grown in modified Mueller-Miller casamino acid medium without beef heart infusion. (4) Tetanus toxin is detoxified with formaldehyde and purified by ammonium sulfate fractionation and diafiltration. Diphtheria and tetanus toxoids are individually adsorbed onto aluminum phosphate.
The adsorbed diphtheria, tetanus and acellular pertussis components are combined with aluminum phosphate (as adjuvant), 2-phenoxyethanol (not as a preservative) and water for injection.
Both diphtheria and tetanus toxoids induce at least 2 units of antitoxin per mL in the guinea pig potency test. The potency of the acellular pertussis vaccine components is determined by the antibody response of immunized mice to detoxified PT, FHA, PRN and FIM as measured by enzyme-linked immunosorbent assay (ELISA).
INDICATIONS AND USAGE
DAPTACEL is a vaccine indicated for active immunization against diphtheria, tetanus and pertussis as a five dose series in infants and children 6 weeks through 6 years of age (prior to 7th birthday). (1)
DOSAGE AND ADMINISTRATION
The five dose immunization series consists of a 0.5 mL intramuscular injection administered at 2, 4, 6 and 15-20 months of age, and at 4-6 years of age. (2.1, 2.2)
DOSAGE FORMS AND STRENGTHS
Suspension for injection, supplied in single dose (0.5 mL) vials (3)
CONTRADINDICATIONS
Severe allergic reaction (e.g. anaphylaxis) after a previous dose of any diphtheria
toxoid, tetanus toxoid, or pertussis-containing vaccine, or any component of
DAPTACEL. (4.1)
Encephalopathy within 7 days of a previous pertussis-containing vaccine with no
other identifiable cause. (4.2)
Progressive neurologic disorder until a treatment regimen has been established and
the condition has stabilized. (4.3)
WARNINGS AND PRECAUTIONS
Carefully consider benefits and risks before administering DAPTACEL to persons with a history of:
– fever ≥40.5°C (105°F), hypotonic-hyporesponsive episode (HHE) or persistent, inconsolable crying lasting ≥3 hours within 48 hours after a previous pertussiscontaining vaccine. (5.2)
– seizures within 3 days after a previous pertussis-containing vaccine. (5.2)
If Guillain-Barré syndrome occurred within 6 weeks of receipt of a prior vaccine containing tetanus toxoid, the risk for Guillain-Barré syndrome may be increased following DAPTACEL. (5.3)
For infants and children with a history of previous seizures, an antipyretic may be administered (in the dosage recommended in its prescribing information) at the time of vaccination with DAPTACEL and for the next 24 hours. (5.4)
Apnea following intramuscular vaccination has been observed in some infants born prematurely. The decision about when to administer an intramuscular vaccine, including DAPTACEL, to an infant born prematurely should be based on consideration of the individual infant’s medical status and the potential benefits and possible risks of vaccination. (5.7)
Syncope (fainting) has been reported following vaccination with DAPTACEL. Procedures should be in place to prevent falling injury and manage syncopal reactions. (5.8)
ADVERSE REACTIONS
Rates of adverse reactions varied by dose number, with systemic reactions most frequent following doses 1-3 and injection site reactions most frequent following doses 4 and 5. Systemic reactions that occurred in >50% of subjects following any dose included fussiness/irritability, inconsolable crying, and decreased activity/ lethargy. Fever ≥38.0°C occurred in 6-16% of US subjects, depending on dose number. Injection site reactions that occurred in >30% of subjects following any dose included tenderness, redness and increase in arm circumference. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Sanofi Pasteur Inc., at 1-800-822-2463 (1-800-VACCINE) or VAERS at 1-800-822-7967 and http://vaers.hhs.gov.
USE IN SPECIFIC POPULATIONS
Pregnancy
DAPTACEL is not approved for use in individuals 7 years of age and older. Human or animal data are not available to assess vaccine-associated risks in pregnancy.
Lactation
DAPTACEL is not approved for use in individuals 7 years of age and older. Human or animal data are not available to assess the impact of DAPTACEL on milk production, its presence in breast milk, or its effects on the breastfed infant.
Pediatric Use
DAPTACEL is not indicated for use in infants below 6 weeks of age or children 7 years of age or older. Safety and effectiveness of DAPTACEL in these age groups have not been established.
Revised: [09/2016]
https://www.vaccineshoppe.com/image.cfm?doc_id=11179&image_type=product_pdf
Tagged Under: Tags: daptacel, Diphtheria and Tetanus Toxoids and Acellular Pertussis Vaccine Adsorbed, dosage, ingredients, insert sheet, side effects, usage, warnings





